Some scientists feel that the d-orbitals lie so high in energy that they should not be treated as valence orbitals but as excited orbitals which cannot confer any appreciable stability to chemical bonds. The normal symbols and used to designate bonding orbitals are started to designate the corresponding anti-bonding orbitalsAbove picture illustrate combination of various AOs the anti-bonding orbital involving the reversal of the sign or signs in one of the orbitals arbitrarily the one of the right-hand side.

Molecular Orbitals Bonding Mcat Organic Chemistry Review
96 on page 393.

. The other is a pie. Describe the orbitals used in bonding and the bond angles in the following compounds. The localized bonding model called valence bond theory assumes that covalent bonds are formed when atomic orbitals overlap and that the strength of a covalent bond is proportional to the amount of overlap.
H 2 CO d. Im gonna start with the Sigma Bond. How to determine which orbitals are used for bonding.
The hybrid orbitals used for bonding by Xe in the unstable XeF2 molecule are orbitals. The shapes predicted by VSEPR. Others feel that while d-orbitals are of high energy in free atoms their energy decreases as other atoms approach to make bonds.
Molecular orbitals for H2. C The 3 s p 2 orbitals orient themselves to get as far from each other as possible resulting in a bond angle of 120. The bonding orbital is used in molecular orbital MO theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule.
Science Chemistry Organic Chemistry 8th Edition Describe the orbitals used in bonding and the bond angles in the following compounds. 1S 2S 2P 32244 and 59 now what actually the Habit orbitals is hybrid orbitals are actually those orbitals which are used for P Bond formation formation so we have to make the electronic configuration of sulphur in ground state so this is three years and this is a free pp ds3p contains hospitals and. Since we know that orbitals with one electron spin are used for covalent bonding any orbitals with both their electrons are represented as lone pairs.
Sn3 c sn3d d sn3 2 sn. The constructive interference causes the electron density between the two atoms to increase. Determine the orbitals used for bonding in the following.
In MO theory electrons are portrayed to move in waves. The results of the wave interference are either bonding σ orbitals σ or anti-bonding σ orbitals σ. CH 3 O b.
B The two sp orbitals point in opposite directionsresulting in a bond angle of 180. Why are hybrid orbitals used in chemical bonding. The new sp3 atomic orbitals on carbon are used to share electron pairs with the 1s orbitals from the four hydrogen atoms as shown in Fig.
Carbon is the central element and assume the double bond to be a single bond for defining geometry BPr 3 NBPr 0 BPr NBPr 3 Trigonal Planar Geometry about Carbon. Who are the experts. This means that the structure is composed of 3sp2 hybrid orbitals and 1 unhybridized p-orbital at the carbon valence level.
When two atomic 1s orbitals combine in the formation of H2 the result is two sigma σ orbitals. Draw the resulting molecular orbitals based on how the electron waves interfere with each other. When more than one of these waves come close together the in-phase combination of these waves produces an interaction that leads to a species that is.
It also assumes that atoms use combinations of atomic orbitals hybrids to maximize the overlap with adjacent atoms. A bonding orbital is formed when two atoms approach each other and the overlap between atomic orbitals results in constructive interference. DONT DRAW ANY LOBES BUBBLES FOR THIS PART b Now draw those atomic orbitals you obtained in part a and then overlap them to form the C-F bonds you only have to draw ONE C-F bond and bonds of the CO.
According to MO theory one sigma orbital is lower in energy than either of the two isolated atomic 1s orbitals this lower sigma orbital is referred to as a bonding molecular orbital. Such as H 2 we use molecular orbitals. To describe the bonding in a homonuclear diatomic molecule A molecule that consists of two atoms of the same element.
That is for a molecule in which two identical atoms interact we insert the total number of valence electrons into the energy-level diagram Figure 919 Molecular Orbital Energy-Level. Because this carbon is also s p to hybridized for the pie bond pie bonds are always made up of p orbitals and therefore you have an overlap of a carbon p with another carbon p. Below are a series of isolated atomic orbitals.
This lowers the kinetic and potential energy of 1 or 2 electrons in the resulting bonding orbital. P x -p orbital p orbital x p p x s s Are there atomic orbitals on C Si N and O Are there atomic orbitals on C Si N and O to accommodate the bonding electrons of to accommodate the bonding electrons of the shapes predicted by VSEPR. S orbital s orbital and and s - s - p.
H 2 CO d. CH 3 O b. Use the two following applets to help you answer the questions below.
All of these are sigma bonds. A The 4 s p 3 orbitals orient themselves to get as far from each other as possible resulting in a bond angle of 1095. At this point lets summarize the bonding in the methane molecule.
Describe the orbitals used in bonding and the bond angles in the following compounds. Describe the orbitals used in bonding and the bond angles in the following compounds. A Use Valence-Bond Theory to explain the shape the angles and the hybridization of the central atom orbitals in the compound below.
As we know only electrons in the outer orbitals are used for bonding. The carbon-carbon bond with a bond length of 154 pm is formed by overlap of one sp3 orbital from each of the carbons while the six carbon-hydrogen bonds are formed from overlaps between the remaining sp3 orbitals on the two carbons and the 1 s orbitals of hydrogen atoms. The signal bond is made up of a carbon S P to orbital overlapping with another carbon S p to orbital.
We review their content and use your feedback to keep the quality high. Send contains 5 orbitals 12345 year in sulphur 3D is Venkat so this is the. Chemistry questions and answers.
Experts are tested by Chegg as specialists in their subject area.

File Quintuple Bond Orbital Diagram2 Png Wikimedia Commons
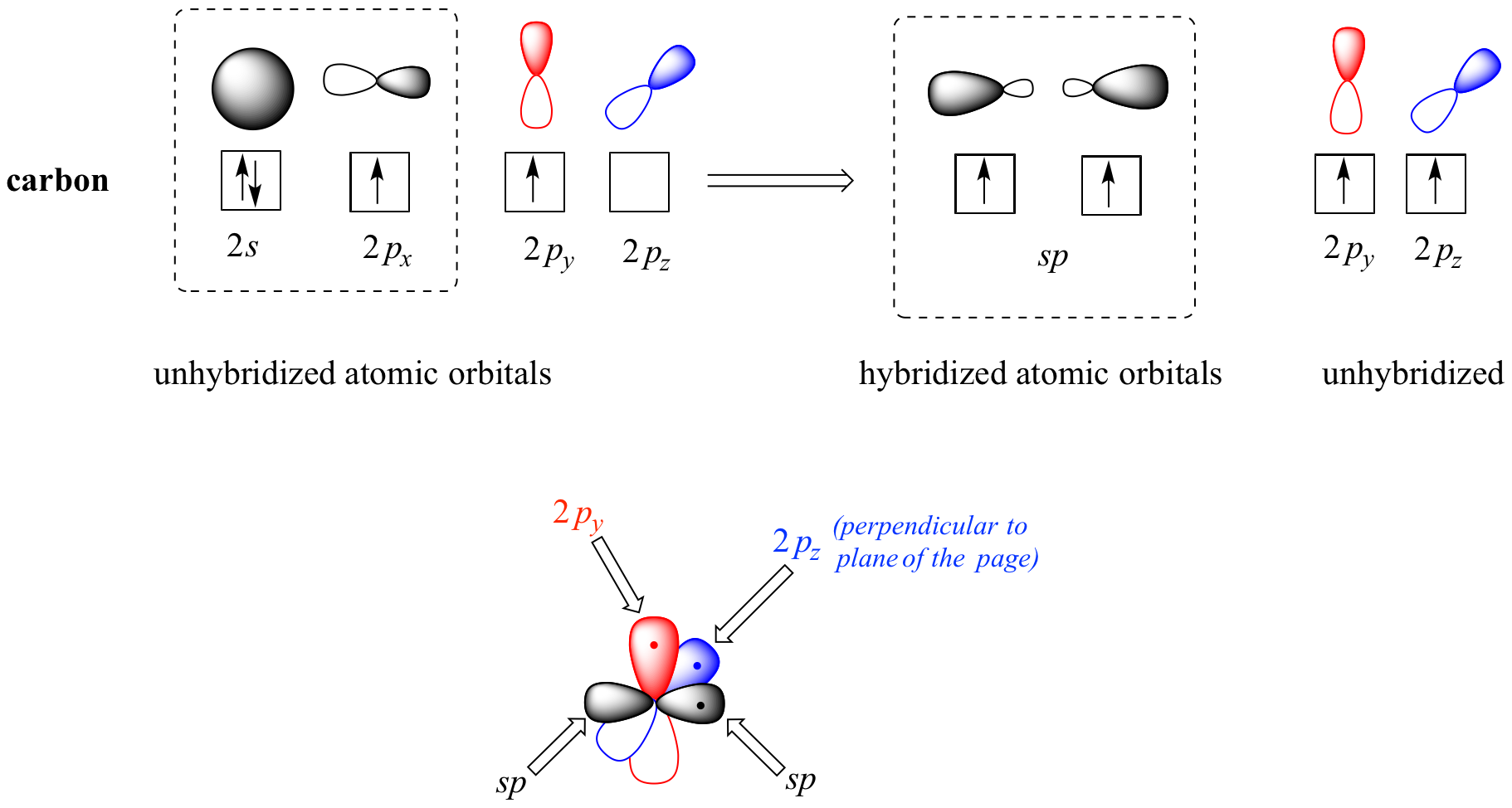
2 2 Hybrid Orbitals Chemistry Libretexts
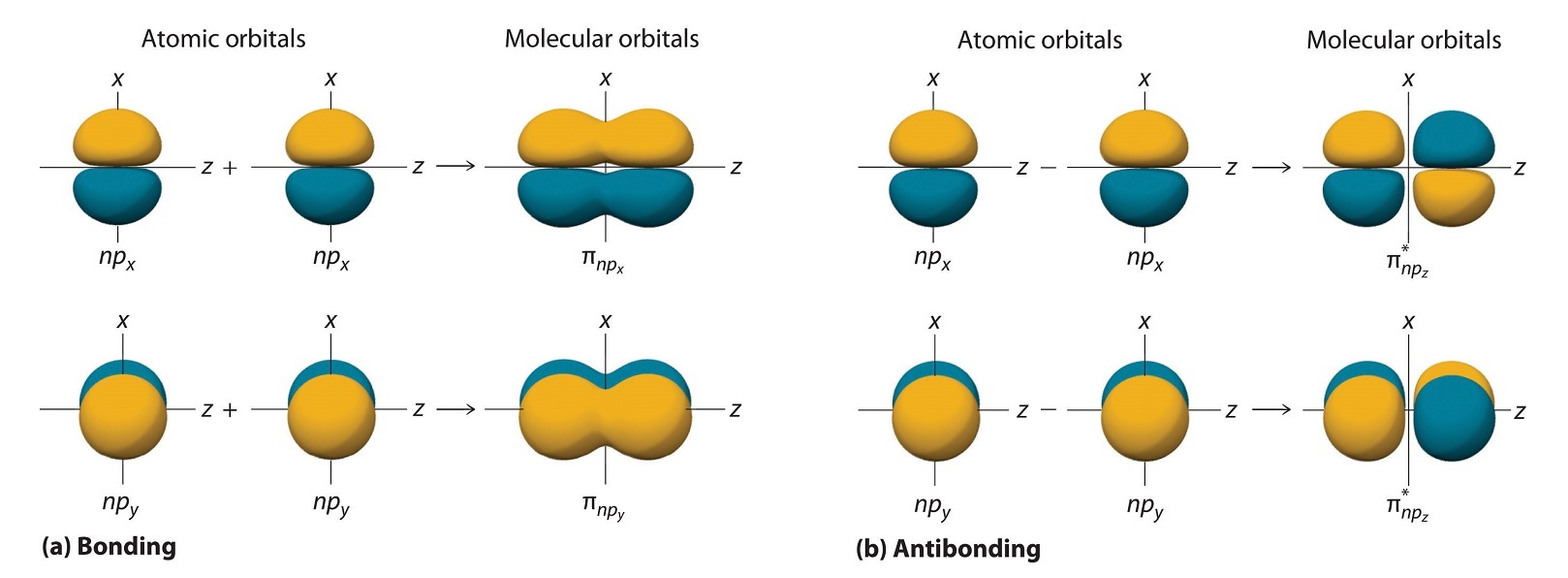
27 Molecular Orbitals With Higher Energy Atomic Orbitals Extra Lecture Chemistry Libretexts
0 Comments